Cosmetic Registration under CDSCO Department

Under the Drugs and Cosmetics Act, 1940 and its associated rules, the regulation of cosmetics in India is structured as follows:
Manufacturing of Cosmetics:
- Regulatory Authority: The manufacture of cosmetics within India is regulated by State Licensing Authorities appointed by the respective State Governments.
- Licensing Requirement: Manufacturers of cosmetics must obtain a license from the State Licensing Authority to legally produce cosmetics. This license is issued after the manufacturing facility and processes are inspected to ensure compliance with the standards set forth in the Drugs and Cosmetics Act and Rules.
- Compliance: The manufacturing facility must adhere to Good Manufacturing Practices (GMP) and maintain the required standards of hygiene, quality control, and safety during production.

Import of Cosmetics:
- Regulatory Authority: The import of cosmetics into India is regulated by the Central Licensing Authority appointed by the Central Government.
- Registration Requirement: Importers of cosmetics must register their products with the Central Licensing Authority before importing them into the country. This registration ensures that imported cosmetics meet the necessary safety and quality standards as per Indian regulations.
- Documentation and Compliance: Importers must submit detailed documentation about the cosmetic products, including their formulation, labeling, and safety data. The Central Licensing Authority reviews this information to determine if the cosmetics are suitable for importation into India.
Key Points:
Definition of Cosmetics: Cosmetics are defined broadly under section 3(aaa) of the Drugs and Cosmetics Act, encompassing articles intended for various cosmetic purposes such as cleansing, beautifying, or altering appearance.
Regulatory Oversight: The regulatory framework ensures that both domestically manufactured and imported cosmetics meet stringent standards for safety, quality, and efficacy
Inspection and Registration: While domestic manufacturers undergo inspection and licensing by State Authorities, importers undergo a registration process overseen by the Central Authority to ensure compliance with Indian regulations.
This regulatory structure aims to safeguard public health by ensuring that all cosmetics marketed in India are safe for human use and conform to established quality standards.
3.Functionalities:-
- Amendment of the Cosmetic Rules, 2020:
Purpose: The amendment process aims to update and modify the existing Cosmetic Rules, 2020 to align with evolving regulatory requirements, industry practices, and consumer safety standards.
Implementation: Amendments are typically proposed by regulatory authorities based on feedback from stakeholders, advancements in scientific knowledge, or changes in international regulatory frameworks.
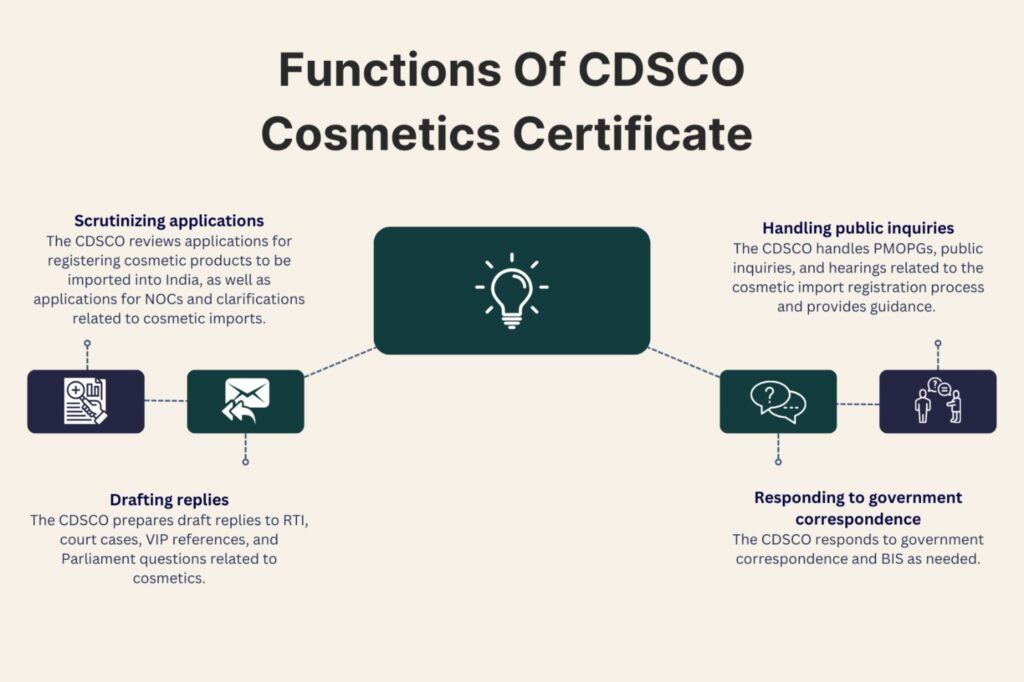
- Scrutiny of Applications for Registration of Cosmetic Products for Import:
Regulatory Requirement: Before importing cosmetics into India, importers must register their products with the Central Licensing Authority.
Scrutiny Process: Applications undergo rigorous scrutiny to ensure compliance with the Drugs and Cosmetics Act, including assessment of product formulation, safety data, labeling, and adherence to Good Manufacturing Practices (GMP).
- Scrutiny of Applications for NOC / Clarifications related to Import of Cosmetic Products:
NOC (No Objection Certificate): Certain cosmetic products may require additional clearances or NOCs, especially those containing specific ingredients or formulations deemed to have potential risks.
Clarifications: Requests for clarifications regarding import requirements are addressed to provide guidance to importers on compliance with regulatory standards.
- Preparation of Draft Replies:
RTI (Right to Information): Drafting responses to RTI queries involves providing accurate and timely information related to cosmetic regulations and processes.
Court Cases, VIP References, Parliament Questions: Drafting responses to legal cases, queries from VIPs, or parliamentary questions requires detailed knowledge of cosmetic regulations and adherence to legal protocols.
4.Who can apply?
According to the “Guidelines to Registration of Imported Cosmetics” issued by the CDSCO (Central Drugs Standard Control Organisation), the following types of Indian entities are eligible to apply for issuance of a registration certificate for imported cosmetics using the prescribed forms COS-1 and COS-2:
Manufacturer (with registered office in India):
- Definition: A manufacturer is a person or entity based outside India who owns the trade name of the cosmetic product and manufactures it either at their own manufacturing site or at a site owned by another manufacturer under their brand name.
- Eligibility: The manufacturer must have a registered office in India.
Authorized Agent of the Manufacturer:
- Definition: An authorized agent is a person or entity in India authorized by the manufacturer to represent them. The agent takes responsibility for all business activities related to the manufacturer’s products in India and ensures compliance with the provisions of the Drugs and Cosmetics Act in all respects.
- Eligibility: The agent must be duly authorized by the manufacturer and operate within the legal framework of the Act.
Subsidiary in India of the Manufacturer:
- Definition: A subsidiary is an entity in India that is wholly owned by the manufacturer based outside India.
- Eligibility: The subsidiary must be owned by the manufacturer and operate within India.
Any Other Importer in India:
- Definition: This category includes any person or entity importing cosmetic products into India who is not the manufacturer, its authorized agent, or its subsidiary.
- Eligibility: This allows for other entities involved in the importation of cosmetics into India to apply for registration of imported cosmetics.
5.Fee Structure for Registration of Cosmetics:
Registration Certificate:
- Fee for grant or retention: $1000 (USD)
This fee is applicable for each category of cosmetic product.
Site Registration:
- Fee: $500 (USD)
This fee is for registering the manufacturing site where the cosmetic products are produced.
- Fee for Each Variant:
Fee: $50 (USD)
This fee applies for each variant of a cosmetic product that is registered.
6.Validity of Registration Certificate (Form COS-2):
A Registration Certificate issued in Form COS-2 remains valid indefinitely (“in perpetuity”), subject to the following conditions:
Retention Fee: Every five years from the date of issue, a retention fee specified in the Third Schedule must be paid to maintain the validity of the certificate.
Suspension or Cancellation: The Registration Certificate may be suspended or cancelled by the Central Licensing Authority if non-compliance with regulatory requirements is found or other valid reasons exist.
Frequently asked questions (FAQs) regarding the registration and import of cosmetics into India, here are the answers provided:
Q.1 What is a Cosmetic in India?
Ans: As per Section 3(aaa) of the Drugs and Cosmetics Act, 1940, Cosmetic means any article intended to be rubbed, poured, sprinkled or sprayed on, or introduced into, or otherwise applied to, the human body or any part thereof for cleansing, beautifying, promoting attractiveness, or altering the appearance.
Q.2 Whether import of Cosmetic is regulated in India?
Ans: Import of cosmetics is regulated in India under the provisions of the Drugs & Cosmetics Act, 1940 & the Cosmetics Rules, 2020.
Q.3 Where can we get a copy of the Gazette notification G.S.R 763 (E) i.e. the Cosmetics Rules, 2020?
Ans: The copy of the Cosmetics Rules, 2020 is available under the Cosmetics section on the CDSCO website.
Q.4 What is the purpose of regulating import of cosmetics in India?
Ans: The import of cosmetics in India needs to be regulated to ensure safety, quality, and performance of cosmetics being imported into India.
Q.5 Who can import cosmetics into India?
Ans: The manufacturer himself/the Authorized Agent of the Manufacturer/the authorized subsidiary of the manufacturer in India/any other importer in India can be an applicant for grant of Import Registration Certificate for import of cosmetics.
Q.6 Which is the Regulatory Authority that governs the regulations of import of Cosmetics into India?
Ans: The Drugs Controller General (India), Central Drugs Standard Control Organization (CDSCO) HQ, Directorate General of Health Services, Ministry of Health and Family Welfare, Government of India, FDA Bhawan, ITO, Kotla Road, New Delhi -110002.

